INTRODUCING THE ACON Biotech
Flowflex™ COVID-19 Antigen Home Test
A rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens
directly
from individuals within 7 days of symptom onset.
The FlowFlex™ COVID-19 Antigen Home Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2, not for any other viruses or pathogens.
The intended use of this
test is for testing twice over two or three days with at least 24 hours and no more than 48 hours between tests.
For use with direct anterior nasal and nasopharyngeal swab specimens, non-invasive test procedures
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Accuracy:
Nasal Swab: 98.8% (95% CI: 97.6%-99.5%) 95% Confidence Intervals
Saliva: 97.1% (95% CI: 94.6%-98.5%) 95% Confidence Intervals - Variant-ready solution
- Rapid results within 15 minutes

THE ACON Biotech
Flowflex™ Home Test Details
A rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens
directly
from individuals within 7 days of symptom onset.
The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s COVID-19 status.
Four key features are:
1 HASSLE FREE
- Easy-to-use nasal swab test
- Requires just 1 test, unlike other COVID-19 antigen home tests that require a 2nd test 2-3 days after the first
2 FAST RESULTS
- Accurate results in 15 minutes
3 SELF CONTAINED
- No need to send off to a lab to obtain results.
- Compact packaging for “On-The-Go” testing
4 FLEXIBLE
- Can be used to test children as young as 2 years old
- For use with and without COVID-19 symptoms

DïGEN LABS
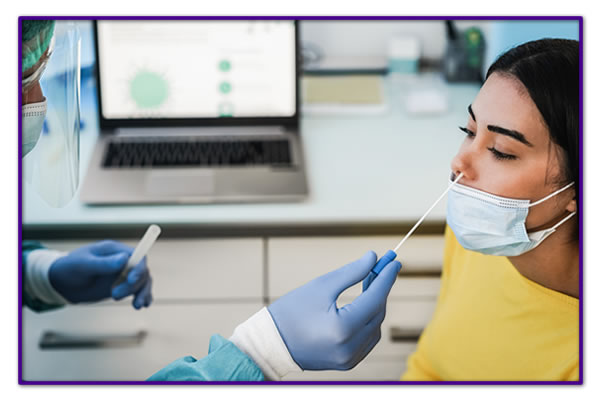
Test Procedure Overview
Blood Wellness panels are groups of tests that determine a patient’s general health.




A rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection.
Qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection.
The Flowflex COVID-19 Antigen Home Test does not require serial testing.
This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens directly from individuals aged 14 years and older or with adult-collected anterior nasal samples directly from individuals aged 2 years or older.
NOTICE
This product has not been FDA cleared or approved but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
MORE COVID TESTING OPTIONS
LEARN MORE ABOUT
DïGEN LABS COVID-19 TESTING PROGRAMS
We are Standing By
CONTACT DïGEN LABS
Contact DïGEN LABS today to see how we can help meet your testing and medical equipment needs.
You don't have to settle for substandard results or frustions associated with dishonest brokers. Find out why not all clinical labs services and medical distributrors are the same.
Get in Touch!
Contact DïGEN LABS. DïGEN LABS is a registered medical device and pharmacuetiucal distributor in the state of Texas. We have direct relationships with manufacturers and various wholesale and reseller outlets.
The Houston Sales Office
DïGEN LABS
11111 Katy Freeway, 910
Houston, TX 77079
Phone: (855) 277-1276
Fax: (888) 636-7540
Email: info@digenlabs.com
Don't wait. CALL NOW!

DïGEN LABS Houston Sales Office
11111 Katy Freeway, 910
Houston, TX 77079
Phone: (855) 277-1276
Fax: (888) 636-7540
Email: info@digenlabs.com
About DïGEN LABS
DïGEN LABS is a registered medical device and pharmacuetiucal distributor in the state of Texas. We have direct relationships with manufacturers and various wholesale and reseller outlets. In addition, we have strong affiliations with high-complexity CLIA and CAP accredited laboratories that specialize in biochemistry, cytogenetics, microbiology, molecular genetics, pharmacology, and toxicology.